The quality control laboratory of Lab M’s facilities in Heywood, UK, has been granted ISO 17025:2005 accreditation by the United Kingdom Accreditation Service (UKAS).
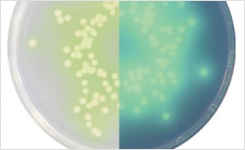
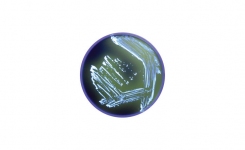
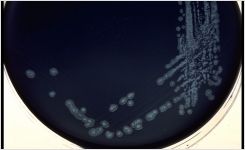
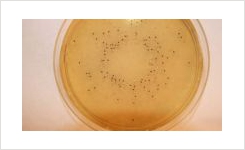
Lab M’s schedule of accreditation covers both the physical and microbiological performance testing of the company’s ready-to-use Pinnacle™ media range. The five methods that were accredited include pH, sterility, fill volume, qualitative performance testing and quantitative performance testing. All methods are based on the new requirements of BS EN ISO 11133:14.
“The requirements of BS EN ISO 11133:14 have been considered throughout the expansion of our Pinnacle range. This newly published standard has been a major focus of all manufacturers and users of culture media,” said Lyn Richards, quality assurance and regulatory affairs manager at Lab M. “The implementation of the new requirements of the standard to all of our QC testing has been a major focus for Lab M over the last 12 months, and to have the methods accredited to ISO 17025 is the next step to demonstrate the quality of the testing of our products. This accreditation will also support the production quality of our ready-to-use media range.”
Lab M’s QC laboratory is a purpose-built facility that is staffed by an experienced team of microbiologists, some of whom have worked with Lab M for more than 20 years. The QC laboratory also has an ongoing investment programme with regards to equipment.
 UKAS is the national accreditation body for the United Kingdom, appointed by government, to assess organisations that provide certification, testing, inspection and calibration services. Accreditation determines the technical competence and integrity of organisations offering testing, inspection, calibration, verification and certification services.
UKAS is the national accreditation body for the United Kingdom, appointed by government, to assess organisations that provide certification, testing, inspection and calibration services. Accreditation determines the technical competence and integrity of organisations offering testing, inspection, calibration, verification and certification services.
In August, Lab M was acquired by Neogen Corporation, a world-wide leader in the development of food and animal safety products and services.
Lab M specialises in the development, manufacture and supply of microbiological culture media and related products. The company is also an established supplier of bulk peptones and other raw materials used in mammalian cell culture and microbial fermentation to produce biomaterials such as vaccines and antibodies. Lab M operates from global headquarters in the UK and is certified in accordance with ISO 9001:2008 and 13485:2003; products for the clinical market are supplied in compliance with the European IVD directive and carry the CE mark. Visit www.labm.com