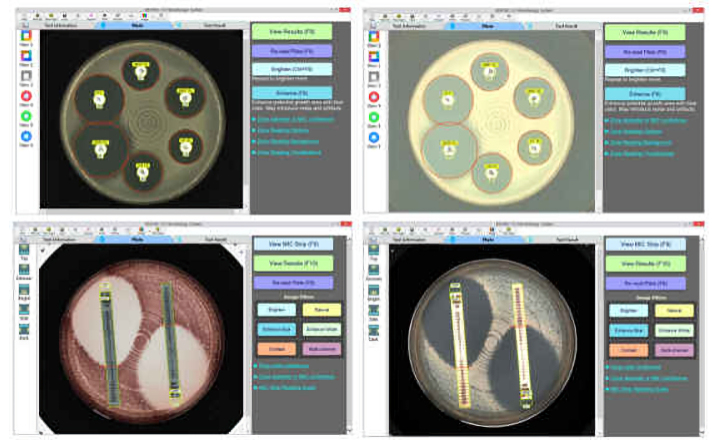
The 2014 CLSI (Clinical Laboratory Standards Institute) M100-S24 guidelines and 2014 EUCAST (European Committee on Antimicrobial Susceptibility Testing) Clinical Breakpoint Tables for Interpretation of MICs and Zone Diameters v 4.0 (1 Jan, 2014) are updated in the BIOMIC V3 software. Changes include new or updated expert rules, updated AST breakpoints, and new or updated QC breakpoints. Disk diffusion and MIC test results are checked for resistance mechanisms, appropriate comments reported, and unlikely results flagged.
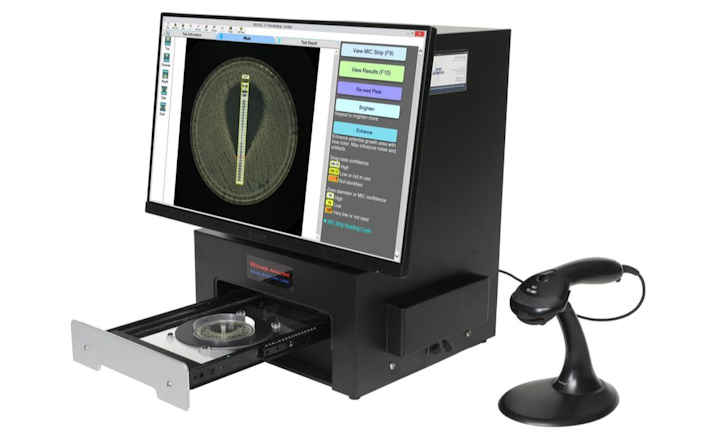
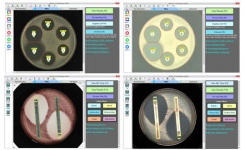
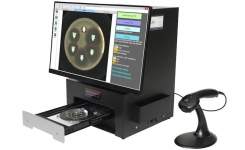
BIOMIC V3’s digital imaging technology automates the reading, interpretation, and reporting of CLSI, EUCAST and BSAC antibiotic disk diffusion tests, Etests (bioMerieux), MIC Test Strips (Liofilchem), 96-well microtiter plates, and identification panels including API (bioMerieux), RapID (Remel), BBLTM Crystal (BD), Liofilchem ID Systems and chromogenic agar plates. Bar code entry and a 2-Way LIS/LIMS interface further speeds lab workflow.