Proficiency Schemes ...
How to Choose the Ri...
17th August 2017 Content supplied by: Campden BRI
Sampling is the Key To Good Microbiology Results
by Dr Phil Voysey, Microbiological Risk Assessment, Campden BRI
Introduction Measuring the microbiological content of foods is of great importance to the manufacturer, retailer, consumer and regulatory authority alike. The taking of examples of the finished product (samples), and testing them for key microorganisms has historically been seen as a measure of the quality of production. There are, however, a number of drawbacks or questions to be answered in adopting this approach such as:
- How many samples should be taken?
- How often should the samples be tested?
- What microorganisms should be tested for?
- What methods should be used?
One major drawback is that the results obtained will only relate to the unit of product sampled – or rather the portion of sample tested. They do not necessarily reflect the remainder of the batch.
Consequently the purpose of testing samples is to show that control systems such as Good Manufacturing Practices and Hazard Analysis & Critical Control Point (HACCP) are working correctly, rather than to show that the food is ‘microbiologically acceptable’. Decisions still need to be made, though, on how to measure quality through sampling, these are reflected in the sampling plan chosen.
Sampling plans In order to assess the microbiological content of a batch of foodstuff, the food has to be sampled. The conditions of this sampling are dictated by the sampling plan. There are two types of sampling plan used in food microbiology - a 2- and a 3-class plan.
For a 2-class plan it is necessary to specify three numbers:
- the number of samples to be tested from the batch, n
- the maximum level for microbiological counts acceptable in the food, m
- the maximum number of these samples which can have microbiological counts above the threshold m, c
Generally 2-class plans are applied to presence/absence determinations. An example of this type of plan can be found in EC Regulation 2073/2005 on microbiological criteria for foodstuffs (Figure 1).
Figure 1. Example of a 2-class sampling plan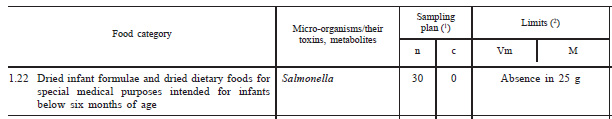
In this sampling plan Salmonella must be absent (not detected) in 30 x 25g samples tested; i.e. n=30, m=0 and c=0.
For a 3-class plan a fourth parameter is defined, M. This defines an unacceptable level of a microorganism in a foodstuff. Three-class plans are normally applied to spoilage or indicator microorganisms where a certain level is permitted. Again, an example can be taken from EC Regulation 2073/2005 (Figure 2).
Figure 2. Example of a 3-class sampling plan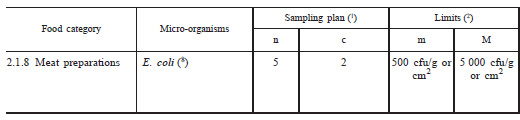
For Escherichia coli in meat preparations, five samples should be tested, all should have levels below 5000 cfu/g, and no more than 2 of the 5 samples are permitted to have counts between 500 and 5000 cfu/g. If these criteria are not met the batch is rejected.
The purpose of an acceptance sampling plan is to accept batches of acceptable quality and to reject batches of unacceptable quality, so in designing or evaluating a plan it is important to have a clear and explicit definition of batch quality.
The Acceptable Quality Level (AQL) defines the minimum quality of a hypothetical borderline batch - distinguishing those that should be accepted from those that should be rejected. The AQL is used to assess the performance of a sampling plan, or the performance required of a plan.
The chance of accepting a batch depends on how good or bad it is when described by the sampling plan operating characteristic. Figure 3 is a plot of quality against probability of acceptance the curve of the graph shows the operating characteristic. There are four possible outcomes:
A) the batch meets the AQL and is accepted: P correct
B) the batch meets the AQL and is rejected: x error
C) the batch fails to meet the AQL and is accepted: x error
D) the batch fails to meet the AQL and is rejected: P correct
Figure 3. Example of a sampling plan operating characteristic showing four outcomes – ‘A’, ‘B’, ‘C’, or ‘D’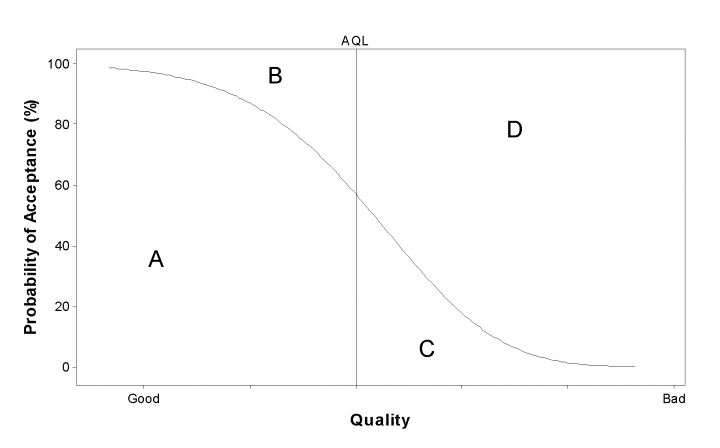
With whichever sampling plan is chosen, some bad batches will be accepted and some good batches rejected. A good sampling plan (in terms of n, m, M and c) should be set to minimise these outcomes. For example, increasing the number of samples examined (n) makes the slope of the operating characteristic steeper, reducing the number of errors.
Choosing a sampling plan is not easy. There is published help available, for example, The International Commission on Microbiological Specifications for Foods (ICMSF), have categorised sampling plans into 15 ‘cases’ as defined by the ‘hazard’, the danger to health posed by the microorganism, and the ‘risk’, the probability that the hazard will be realised, (Figure 4).
Figure 4. Examples of sampling plans suggested by ICMSF (1986, 2002)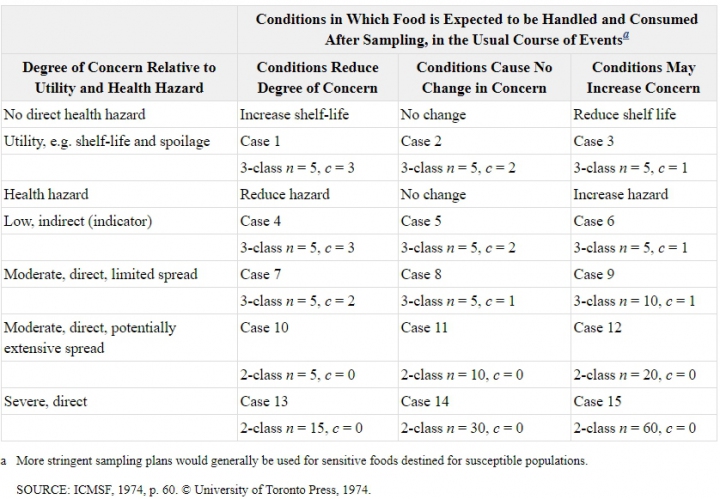
Final Comments Alongside the adoption of techniques such as HACCP, Microbiological Risk Assessment and Statistical Process Control, sampling is a useful monitoring tool to assist in the production of safe food. The choice of which sampling plan to use is difficult, especially as all are flawed. However, assistance and guidance is available for choosing the best plan for a given situation.
Campden BRI have considerable experience in helping companies define sampling plans and continue to research the area to ensure plans operate efficiently and yield meaningful results.
References
- International Commission on Microbiological Safety of Foods (ICMSF) (1986) Microorganisms in foods 2. Sampling for microbiological analysis: principles and specific applications. University of Toronto Press. Now out of print, but superseded by ICMSF Microorganisms in Foods 7: Microbiological Testing in Food Safety Management, (2002) New York:Kluwer Academic/Plenum Publishers
- Campden BRI (2001) Designing and improving acceptance sampling plans – a tool. Review No. 27.

About the author: Dr Phil Voysey is an experienced, well–respected food microbiologist with 'all round' knowledge and ability in food manufacturing and research environments.
Currently Phil is a Section Manager in the Microbiology Department at Campden BRI (Chipping Campden) and his duties involve organising and running microbiology training courses and the Campden Microbiology Proficiency Scheme. Speciality areas of microbiology include: Listeria; Yeasts; Moulds; Microbiological Risk Assessment; Microbiological Criteria; and Cereals & Milling Microbiology. Phil has written numerous papers and contributed towards a variety of articles and publications and been invited to present at many International conferences.
Tags:
Date Published: 17th August 2017
Source article link: View
Proficiency Schemes for Microbiology Laboratories
How to Choose the Right

