Bio-Rad Launches a N...
Bio-Rad Launches iQ-...
4th July 2018 Content supplied by: Bio-Rad Laboratories
RAPID’B.cereus Detects and Counts All Pathogenic B. cereus in Foods
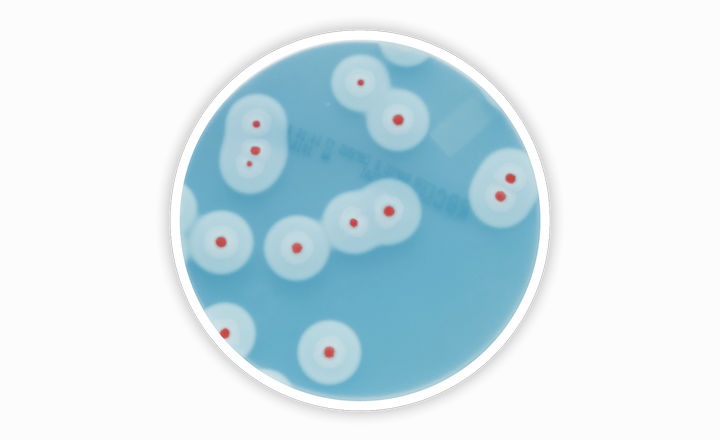
The Bacillus cereus group comprises prolific organisms that are widely distributed in the environment. Some species of this group can be pathogenic to humans and/or animals, but only B. cereus sensu stricto and B. cytotoxicus have been described as food poisoning agents and present a risk for human health. Thus, B. cereus is considered as a “process hygiene criteria” (Quality Indicator) by different national or international regulations or professional federations and should be quantified in different food matrices.
The ISO standard method (ISO 7932) for enumeration of B. cereus uses Mannitol Egg Yolk Polymyxin (MYP). This standard is currently under revision. Food matrices with significant background flora, like spices or raw vegetables, are challenging samples for MYP media. Due to the poor selectivity of this media, the background flora is not sufficiently inhibited and can spread onto the agar surface making the enumeration of B. cereus most of the time fastidious and sometimes impossible. In contrary, agar media having a high selectivity and inhibiting background flora, do not allow the growth of sensitive B. cereus strains like B. cytotoxicus and result in an underestimated enumeration of the targeted bacteria. Finding a good compromise between the needs of selectivity and sensitivity is always a balancing exercise. It is exactly what the new chromogenic media developed by Bio-Rad achieves.
RAPID’B.cereus is the newest edition to the RAPID chromogenic media family. Based on a chromogenic principle and the detection of phospholipase activity, it allows the detection and the enumeration of B. cereus strains. B. cereus colonies appear red surrounded with an opaque halo. The selected nutritive mixture permits optimal growth of all the members of the B. cereus group in less than 24 hr and no confirmation step is required. At the same time, the background flora is inhibited by the selective mixture contained in the agar, even when highly contaminated matrices are tested. This selective mixture also prevents the spreading of rhizoid colonies like B. mycoides.
Combining strong coloration of typical colonies and the contrast of the halo, accurate counting is very easy to perform. The delicate balance of selective and nutritive agents also allows for clear enumeration of the sensitive B. cytotoxicus. RAPID’B.cereus gives users confidence that they are enumerating all pathogenic B. cereus strains.
The media, already available as ready-to-use Petri dishes, has also been developed in dehydrated format with supplements (soon available) allowing the use of either a plate surface inoculation protocol or pour plate protocol. In order to optimize laboratory workflow, the plates can be stored at 2 – 8 °C for 72 hr prior to the reading step.
External studies performed by independent third party expert laboratories have demonstrated that the performance of the new media is equivalent to the gold standard but in a shorter time and without extra work required reading difficult plates when raw samples are tested. Bio-Rad has started the validation of this new media according to the ISO 16140-2 standard.
Tags:
Date Published: 4th July 2018
Related news
Bio-Rad Launches a New PCR
Bio-Rad Launches iQ-Check Vibrio Real-Time


