Alpha Labs New Later...
Make Your Mycotoxin ...
2nd May 2019 Content supplied by: Micronic BV
Micronic Receives Environmental Impact Label for 2D Data-Matrix Coded Screw-Cap Tubes
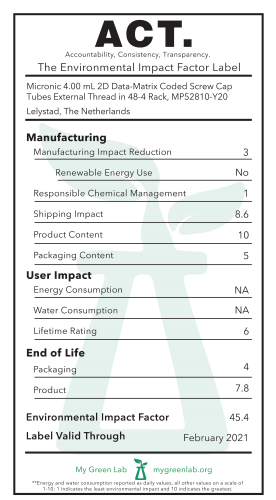 Micronic has joined an elite group of manufacturers committed to product transparency and sustainable procurement. Two of Micronic’s most popular 2D Data-Matrix Coded Screw-Cap Tubes, the 1.40 mL and the 4.00 mL, have received an ACT label.
Micronic has joined an elite group of manufacturers committed to product transparency and sustainable procurement. Two of Micronic’s most popular 2D Data-Matrix Coded Screw-Cap Tubes, the 1.40 mL and the 4.00 mL, have received an ACT label.
ACT is the first environmental impact factor label for laboratory products, developed by the non-profit My Green Lab. ACT—which stands for accountability, consistency, and transparency—is designed to provide critical information on the environmental impact of laboratory products.
The environmental impact of laboratories is enormous. Buildings with laboratories consume as much as eight times more energy than office buildings and can use millions of gallons of water per year. In 2014 alone, it was estimated that labs discarded over 12 billion pounds of plastic—which doesn't include gloves, hazardous waste and packaging waste. But it doesn’t have to be like this. Through smarter purchasing, organizations and individuals can significantly reduce their environmental impact. By providing third-party verified information on a product’s energy consumption, water use, and end-of-life, manufacturers with an ACT label empower purchasers to consider sustainability alongside performance.
“Micronic’s participation in the ACT program demonstrates a commitment to their customers and to the planet,” says Allison Paradise, CEO of My Green Lab. “Micronic’s leadership will enable their customers to make smarter, more sustainable choices.”
Product labels can be found on Micronic’s website as well as on the ACT website.
More information on the ACT label program can be found at act.mygreenlab.org.
Tags:
Date Published: 2nd May 2019
Source article link: View
Related news
Alpha Labs New Lateral Flow
Make Your Mycotoxin Analysis Easier






