InSite - Fast Food Pathogen Detection
Manufacturer: Hygiena International
Principle: Selective chromogenic media
Approvals: InSite Salmonella and InSite Listeria broth is AOAC approved
Suitability: Surface environmental monitoring
Capital equipment required: Incubator
Description:
Key Points:
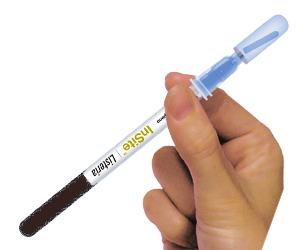
InSite is a self-contained, reagent swab device for the detection of pathogens in environmental samples. Each device contains a chromogenic liquid media formulated with antibiotics, growth enhancers, and colour changing compounds. The devices consist of pre-enrichment media and neutralisers on the swab and selective enrichment in the separate bulb. Specific version for Listeria and Salmonella give presumptive positive results in ~30 hours.
Key Points:
- Tests Available:
- InSite Listeria spp.
- InSite Salmonella
- Large swab bud to enhance sample collection
- Simple test procedure
- Easy to interpret colour change results
- Presumptive positive results in 24 – 30 hours
- Negative results provided in 48 hours
- Self-contained tube reduces risk of cross contamination
Areas:
Tags:
Company contact details:
Hygiena view full details
Watford
Herts
United Kingdom
Website: Visit Website
Tel: +[44] 1923 818821
Hygiena view full details
Watford
Herts
United Kingdom
Website: Visit Website
Tel: +[44] 1923 818821






