Limulus Color KY Ser...
26th February 2015 Content supplied by: Fujifilm Wako Chemicals U.S.A Corporation
PYROSTAR™ ES-F – Endotoxin Specific LAL Products
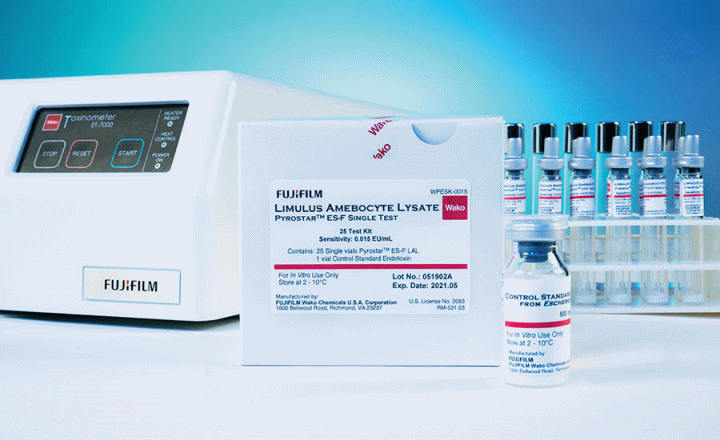
The PYROSTAR™ ES-F series of reagents are endotoxin-specific and specially formulated to be unreactive to (1,3)-ß-D-glucan. In addition, these products are dual-purpose, meaning they are formulated to be used as either a gel clot or kinetic turbidimetric assay. The kits are available in a single-test or multi-test configuration and are matched with a specific Control Standard Endotoxin (CSE). PYROSTAR™ ES-F reagents work well with samples that have color or with samples that present difficulties in quantification by the KCA.
Single-test vials come with pre-dispensed LAL reagent for a single measurement. They are designed to be used with our Toxinometer® Measurement System (Toxinometer®) to assist labs transitioning from a gel clot to a kinetic turbidimetric methodology and is ideal to assay a small number of samples or for a less-experienced user.
Key Features:
- Endotoxin-specific reagent avoids false-positive results from glucans
- Gel clot sensitivities include 0.015 EU/mL, 0.03 EU/mL, 0.125 EU/mL and 0.25 EU/mL
- KTA sensitivities range from 0.001 to 10 EU/mL
- Can be used with the traditional water bath or heat block
- Can be used with the Toxinometer® to facilitate easier transition from gel clot to KTA
The multi-test vial is designed to be utilized with a water bath, heat block, or the Toxinometer® and is ideal for a user testing a larger number of samples.
Key Features:
- Endotoxin-specific reagent avoids false-positive results from glucans
- Dual-purpose reagent can perform either a gel clot or kinetic turbidimetric assay (KTA)
- Gel clot sensitivities range from 0.015 to 0.25 EU/mL
- Kinetic turbidimetric sensitivities range from 0.001 to 10 EU/mL
- Due to its high sensitivity, chances of interference are reduced
- Facilitates easier transition from gel clot to KTA
Tags:
Date Published: 26th February 2015
Source article link: View
Limulus Color KY Series Can


