Merck Millipore Exhi...
New Additions to the...
15th June 2015 Content supplied by: Merck Millipore
How Merck Millipore Can Change Your Food Pathogen Workflow
There doesn’t seem to be any let up in the number of food poisoning outbreaks with headline grabbing recalls; Salmonella in leafy greens and beansprouts, Listeria in Blue Bell ice-cream and caramel apples, the list goes on.
In this interview with rapidmicrobiology.com, Stephen Kuchenberg, Technology Manager-Food Market at Merck Millipore discusses how the Merck Millipore range of culture media can help streamline the workflow for laboratories testing for Salmonella and Listeria in foods.
According to Kuchenberg, the current big focus area particularly in North America is monitoring for Listeria, for example in poultry carcass rinses, recent legislation has led to a dramatic rise in the amount of testing. Merck Millipore have been producing culture media for over 120 years and have a complete portfolio of media for all types of food pathogen testing including Listeria and Salmonella. The range includes most standard broths and agars whether it’s ISO, FDA-BAM or FSIS MLG formulations that are required.
Kuchenberg explains what makes the Merck Millipore dehydrated culture media different, “all our dehydrated media are presented as granules rather than as a powder, this greatly reduces the amount of atmospheric dust in the lab, avoiding health and safety issues and also increasing the accuracy of the prepared media as the granules dissolve more easily rather than forming a clump,” Kuchenberg illustrates this very well by adding “if you imagine yourself in the kitchen at home, which is easier to dissolve and not end up with lumps – flour or granulated sugar?”.
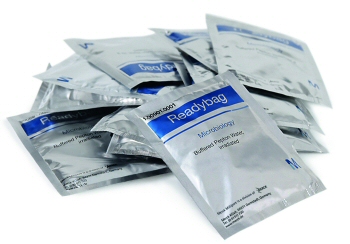
Whilst there are various rapid assays on the market for food pathogen testing, there are many labs who still rely on the conventional media based approach, and Kuchenberg has observed that “the smaller customer is not going to adopt these more expensive, more sophisticated technologies if it doesn’t make sense for them”. However these labs still need a solution that improves their workflow and allows them to cope with fluctuating testing demands. “It’s this type of lab that are ideally suited for the recently introduced Merck Millipore Readybag® range.”
Readybag pouches contain pre-weighed, irradiated Merck Millipore granulated media. The entire contents of the pouch are added to the sample in a stomacher bag and re-hydrated with sterile, filtered water. There is no need for autoclaving or the addition of any additional supplements. With a long shelf life of 3 years, the bags can be stored in the laboratory ready to go, so the lab can cope with a sudden large influx of tests, without needing to wait for primary enrichment media to go through preparation and QC holding.
The range currently consists of Buffered Peptone Water (BPW) for Salmonella pre-enrichment and Half-Fraser Broth for Listeria primary enrichment in standard sizes for testing 25g samples. Also for the larger pooled samples there are 86g packs BPW for Salmonella testing in 375g composite samples and 63g Half Fraser Broth packs for Listeria testing in 125g composite samples.
Kuchenberg has found that “the Readybag has proved itself ideally suited to dairy and meat testing labs where they are testing pooled samples, for example 375g requires 3,375 ml Buffered Peptone Water - and that is a lot of media to autoclave!”
“For labs that make media traditionally rather than using an automated media prep system, Readybag is the most sensible option especially if they have a relatively high throughput of tests and are handling these larger composite samples.”
Using Readybag changes the workflow of a lab by eliminating the media kitchen and QC step. Unlike other ready-to-rehydrate media in a bag, Readybag is designed to be for single sample use so there’s no left over, unused media – it’s ‘one sample, one bag’. Also with the ready-to-rehydrate bags there is still only 20 Litres available in each bag so they are not sufficient for high throughput labs needing these large volumes of BPW. Kuchenberg observes that “Those labs that have adapted to the Readybag have greatly improved their workflow, so whilst the media cost per test may be a bit more, the operational costs of running the lab are down.”
Following on from recall notices, laboratories can find themselves suddenly having a large influx of samples for food pathogen testing, having a flexible on-demand source of media can be key to being able to cope with a variable number of tests.
Introducing a change to the normal lab testing routine can be time consuming in itself but Merck Millipore have specialists who can help “In addition to the industry standard customer service and technical specialists who help a lab to adapt to new workflows, we also have an applications development group in Darmstadt who will work with customers on non-standard applications.” The Merck Millipore granular media including Readybag is guaranteed to be fully compliant with ISO 11133*.
Microbiological culture media is not going away any time soon, even with the newer technologies there is still normally an enrichment step involved “Microbiology is still microbiology, it’s always going to require at least in the near future, culture media and classical methods” says Kuchenberg.
Click here for more information on the Merck Millipore granulated media range and Readybag for Salmonella and Listeria enrichment broths.
Stephen Kuchenberg was talking to Sue Brockman, editor at rapidmicrobiology.com
*ISO 11133 is the international standard relating to the preparation, production, storage and performance testing of culture media, Readybag will be fully compliant as of fall 2015.
Watch the Merck Millipore culture media range video - https://www.youtube.com/watch?v=mnsqhBxBxAU Watch the Merck Millipore Readybag video - https://www.youtube.com/watch?v=Yvemezh8bPo
Tags:
Date Published: 15th June 2015
Source article link: View
Related news
Merck Millipore Exhibits at ASM 2015
New Additions to the MAS-100&


