Pipetting Tips - str...
Automated Hepatitis ...
25th October 2016 Content supplied by: ELITechGroup
Enterobacterial Resistance to Polymyxin Detected by Colour Change Test
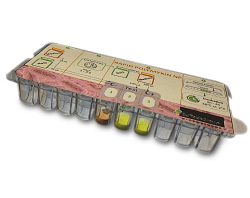
Among the most clinically significant multidrug-resistant bacteria are carbapenemase-producing Enterobacteriaceae. Because these bacteria usually remain susceptible to polymyxins, interest in this old class of antibiotics has been recently renewed. However, the increasing use of colistin explains why Enterobacterial strains resistant to colistin are increasingly reported worldwide.
Currently available polymyxins susceptibility methods are fastidious, time-consuming (24 hours) and some methods are not reliable. They are poorly adapted to the clinical need and to the prevention of the dissemination of those multidrug resistant isolates.
ELITechGroup Microbiology have now released its new Rapid Polymyxin NP test, a rapid, reliable and cost-effective test to detect polymyxin (polymyxin E or Colistin, polymyxin B) resistant Enterobacteriaceae.
This test is based on the detection of the glucose metabolization related to bacterial growth in presence of a defined concentration of colistin. Formation of acid metabolites consecutive to the glucose metabolization is evidenced by a color change (orange to yellow) of a pH indicator.
The Rapid Polymyxin NP test is easy-to-perform, very sensitive and specific. It can detect in only a couple of hours polymyxin-resistant and –susceptible isolates from any enterobacterial species, regardless of the molecular mechanism of polymyxins resistance.
This test offers the possibility of detecting polymyxins resistance from bacterial cultures before any antimicrobial drug susceptibility testing results are obtained. Results are obtained at least 16 hours sooner with this test than with the reference broth microdilution method. It is as reliable as the reference dilution technique but much less cumbersome and is not based on diffusion of large polymyxin molecules in agar, which therefore prevents false susceptibility results.
Tags:
Date Published: 25th October 2016
Source article link: View
Related news
Pipetting Tips - straight as
Automated Hepatitis Viral Load Testing






