For Better Results T...
Understanding M40-A2...
4th November 2014 Content supplied by: MWE
EBOLA or FLU? As the Seasons Collide Which Would you Choose?
Prevent avoidable deaths this winter.
While the focus is predominantly on the rapid spread of the Ebola virus, the flu season is now gearing up in some parts of the world such as the United States and other countries await its anticipated arrival.
Both Ebola and Influenza can be deadly, but with flu comes the readily available vaccine offering protection & ultimately preventing the spread of the disease.
When comparing the two viruses people are at much higher risk of becoming infected with the Influenza virus. Unlike Ebola, Influenza is airborne and therefore highly contagious as its transmission is passed on by coughing, sneezing and through the contamination of hands and surfaces exposed to the infected virus particles.
Initial symptoms of both viruses appear to be very similar: cough, sore throat, fatigue, muscle aches and an increased temperature, but in most cases after 8-12 days signs of the flu illness begin to subside but the symptoms of Ebola can intensify dramatically.
As stated by the Centre for Disease Control and Prevention, Influenza poses far greater health risks worldwide than Ebola and it estimates that tens of thousands will die each year from the Influenza virus.
 So be prepared this winter; Influenza? Ebola? RSV? Norovirus? Rhinovirus?
So be prepared this winter; Influenza? Ebola? RSV? Norovirus? Rhinovirus?
Whilst it is impossible to predict what the 2014/2015 flu season will bring about, due to the timing, severity and the length of the season, one thing is predictable;
MWE are prepared for all eventualities with a full range of Virology products for all your needs.
See our full range of Virology products by clicking here.
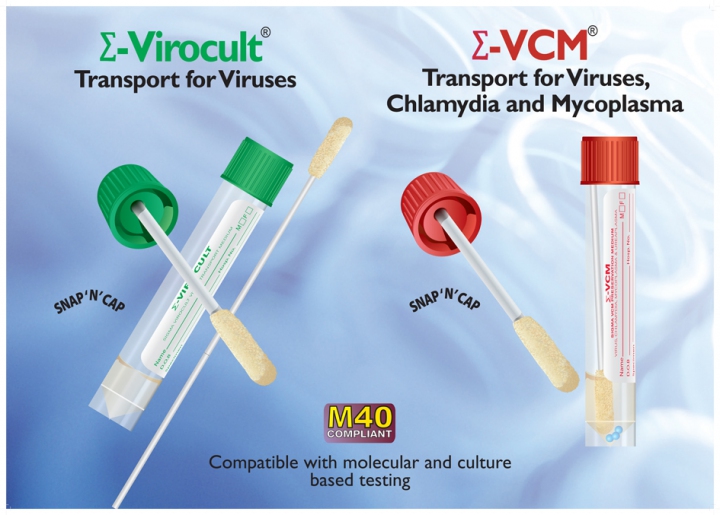
MWE’s Sigma Virocult® has long been recognised as the leading viral transport device in both the clinical and also the veterinary market. Demonstrating the survival of numerous viruses at ambient temperatures Sigma Virocult® has proven to be the number one choice by Virology departments worldwide.
Our highly referenced product Sigma VCM™ is a Universal Transport Medium which provides collection and transport for Viruses, Chlamydia, Mycoplasma and Ureaplasma. Retaining all of the qualities of Virocult®, Sigma VCM™ allows survival and recovery of target organisms, whilst antimicrobials prevent growth of contaminating bacteria or fungi in the specimen. Read more about our product range by clicking here.
Offering exceptional convenience, both Sigma Virocult® and Sigma VCM™ offer a choice of vial size, fill capacity, different swab types (Flock or Foam) and bud sizes. Formats are readily available with up to 3 swabs for the sampling of multiple sites. Both transport devices are fully compatible with cell culture and molecular testing systems.
 Our swabs have a soft touch buds, (either polyurethane foam or PurFlock® polyester), highly absorbent but gentle for sampling from sensitive or inflamed areas. MWE’s range of viral transport products are ready to test on arrival in the laboratory & of course are all fully M40 compliant. The systems have been validated in accordance with CLSI’s M40-A 1 standard for the recovery of live virus, including influenza virus for 96 hours at both room and refrigeration temperatures, while additional studies show that nucleic acid materials are preserved intact for at least 14 days.
Our swabs have a soft touch buds, (either polyurethane foam or PurFlock® polyester), highly absorbent but gentle for sampling from sensitive or inflamed areas. MWE’s range of viral transport products are ready to test on arrival in the laboratory & of course are all fully M40 compliant. The systems have been validated in accordance with CLSI’s M40-A 1 standard for the recovery of live virus, including influenza virus for 96 hours at both room and refrigeration temperatures, while additional studies show that nucleic acid materials are preserved intact for at least 14 days.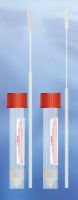
Sigma Virocult® and Sigma VCM™ are both CE-marked as Class IIa medical devices (MDD & IVD), and are available through our distributors in most countries.
As the winter months can prove unpredictable, it is vital to ensure adequate stock levels of Sigma Virocult® & Sigma VCM™ are maintained.
MWE’s production teams are very capable of responding quickly to any surges in demand. However it is important that you remain prepared with sufficient stock levels to carry you through the initial phase, should such a surge occur.
DONT BE CAUGHT OUT THIS WINTER – ORDER YOUR STOCKS NOW:
Call us on: +44 (1225) 810361 (option 1) Fax us on: +44 (1225) 810153
1. CLSI, 2003, Quality Control of Microbiological Transport Systems: Approved Standard NCCLS Document M40-A
® PurFlock® is a registered trademark of Puritan Medical Products Co. LLC
Further details from
Visit us at Booth 1F10 at Medica 12-15 November, Dusseldorf
Tags:
Date Published: 4th November 2014
Source article link: View
Related news
For Better Results Tomorrow, Choose
Understanding M40-A2. Are Your


